Cardiovascular Regenerative Medicine
About us
We investigate various research subjects in cardiology, including heart development, aging, heart failure, ischemia, arrhythmia as well as regenerative medicine, with embryological, molecular biological, physiological, and pharmacological approach.
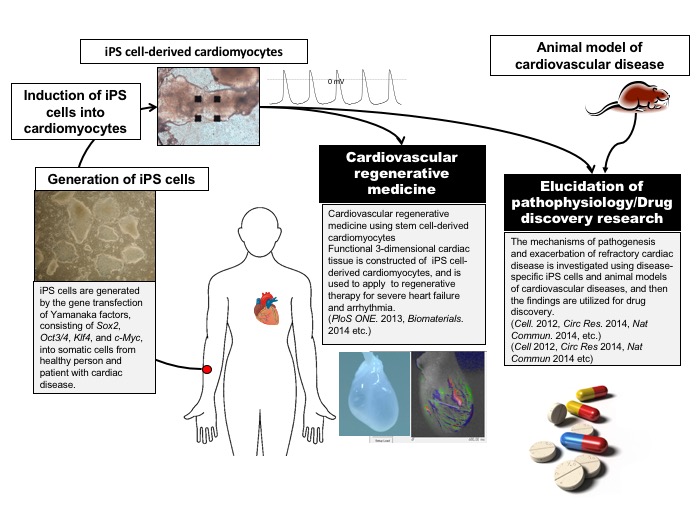
As for regenerative area of these themes, we’re also involved in several collaborative projects with Department of Cardiovascular Surgery, Osaka university graduate school of medicine, and address translational research on cardiovascular regenerative therapy to aim for the clinical application in a few years. In particular, we undertake a cell- and tissue-based therapy for bradycardias (which is defined as a ‘biological pacemaker’) as well as a preclinical study on severe ischemic cardiomyopathy with stem cell-, mainly induced pluripotent stem cell (iPSC)-, derived cardiomyocytes. In addition, we explore a novel approach for heart failure and arrhythmia through the regulation of autonomic function with regenerative method.
Another main subject of regenerative medicine in our research group is about disease-specific iPSCs. We generate disease-specific iPSCs from the patients with hereditary cardiomyopathy, including Noonan syndrome, Fabry’s disease, and Duchenne type Muscular Dystrophy, and differentiate the iPSCs into cardiomyocytes, and then analyze the character of the cardiomyocytes in order to elucidate the underlying mechanism and to explore a novel therapy for these disorders.
Other than regenerative research as mentioned above, we also address the following themes: to reveal the role of Wnt signaling, which is one of differentiation factors, on cardiovascular disease, senescence, and inflammation; to discover the regulatory mechanism of cardiac fibrosis and to develop a new therapy for heart failure targeting the cardiac fibrosis; to elucidate stress response in cardiomyocytes focusing on molecules involved in the regulation of aging and metabolism.
Please click here to the website linked to advanced cardiovascular regenerative medicine共同研究講座
Research objective and content
Research objective
To elucidate the underlying mechanism of cardiovascular disease using regenerative medical, embryological, molecular biological, physiological, and pharmacological method, and to develop a novel therapy for cardiovascular disease.
Research content
- Regenerative research in cardiovascular disease: to develop a novel therapy for heart failure and arrhythmia using regenerative medical and tissue engineering technique.
- To analyze the underlying mechanism of heart failure using disease-specific iPSCs and model mice of heart failure, and to explore the new treatment for heart failure.
- To reveal the role of Wnt signaling on cardiovascular disease, aging (senescence), and inflammation
- To examine the mechanism of cardiac fibrosis.
- To investigate stress response in cardiomyocytes focusing on molecules involved in the regulation of aging and metabolism.
Member
- Specially-appointed associate professor
- Jong-Kook Lee, MD, PhD
- Specially-appointed assistant professor
- Keiko Miwa, PhD
- Medical staff
- Taku Sakai, MD
- Medical staff
- Tomoaki Higo, MD
- Graduate student (doctoral course)
- Akira Yoshida, MD
Teruki Yokoyama, MD
Yuki Kuramoto, MD
Masato Shibamoto, MD
Hiroyuki Nakanishi, MD
Satoki Tomoyama, MD
Jun Lee, MD
Hideki Yasutake, MD - Technical assistant
- Chikako Kageyama
- Clerical assistant
- Kyoko Ishino
Publications
- Wang Q, Oka T, Yamagami K, Lee JK, Akazawa H, Naito AT, Yasui T, Ishizu T, Nakaoka Y, Sakata Y, Komuro I. An EP4 receptor agonist inhibits cardiac fibrosis through activation of PKA signaling in hypertrophied heart. Int Heart J. 2016 (In press)
- Yano M, Akazawa H, Oka T, Yabumoto C, Kudo-Sakamoto Y, Kamo T, Shimizu Y, Yagi H, Naito AT, Lee JK, Suzuki J, Sakata Y, Komuro I. Monocyte-derived extracellular Nampt-dependent biosynthesis of NAD(+) protects the heart against pressure overload. Sci Rep. 2015 Nov 2;5:15857.
- Hashimoto A, Naito AT, Lee JK, Kitazume-Taneike R, Ito M, Yamaguchi T, Nakata R, Sumida T, Okada K, Nakagawa A, Higo T, Kuramoto Y, Sakai T, Tominaga K, Okinaga T, Kogaki S, Ozono K, Miyagawa S, Sawa Y, Sakata Y, Morita H, Umezawa A, Komuro I. Generation of Induced Pluripotent Stem Cells From Patients With Duchenne Muscular Dystrophy and Their Induction to Cardiomyocytes. Int Heart J. 2016 Jan 19;57(1):112-7.
- Yamagami K, Oka T, Wang Q, Ishizu T, Lee JK, Miwa K, Akazawa H, Naito AT, Sakata Y, Komuro I. Pirfenidone Exhibits Cardioprotective Effects by Regulating Myocardial Fibrosis and Vascular Permeability in Pressure Overloaded Hearts. Am J Physiol Heart Circ Physiol. 2015 Jun 8: ajpheart.00137.2015. doi:10.1152/ajpheart.00137.2015.
- Okada K, Naito A.T., Higo T, Nakagawa A, Shibamoto M, Sakai T, Hashimoto A, Kuramoto Y, Sumida T, Nomura S, Ito M, Yamaguchi T, Oka T, Akazawa H, Lee JK, Morimoto S, Sakata Y, Shiojima I, Komuro I. Wnt/b-catenin signaling contributes to skeletal myopathy in heart failure via direct interaction with forkhead box o. Circ Heart Fail. 2015;8:799-808.
- Sumida T, Naito A.T., Nomura S, Nakagawa A, Higo T, Hashimoto A, Okada K, Sakai T, Ito M, Yamaguchi T, Oka T, Akazawa H, Lee JK, Minamino T, Offermanns S, Noda T, Botto M, Kobayashi Y, Morita H, Manabe I, Nagai T, Shiojima I, Komuro I. Complement C1q-induced activation of b-catenin signalling causes hypertensive arterial remodeling. Nature Communications. 2015 (DOI: 10.1038/ncomms7241)
- Kawamura T, Miyagawa S, Fukushima S, Yoshida A, Kashiyama N, Kawamura A, Ito E, Saito A, Maeda A, Eguchi H, Toda K, Lee JK, Miyagawa S, Sawa Y. N-glycans: phenotypic homology and structural differences between myocardial cells and induced pluripotent stem cell-derived cardiomyocytes. PLoS One. 2014 Oct 30;9(10):e111064. (doi:10.1371/journal.pone.0111064.)
- Yasui H, Lee JK, Yoshida A, Yokoyama T, Nakanishi H, Miwa K, Naito AT, Oka T, Akazawa H, Nakai J, Miyagawa S, Sawa Y, Sakata Y, Komuro I. Excitation propagation in three-dimensional engineered hearts using decellularized extracellular matrix. Biomaterials. 2014;35(27):7839-50.
- Kudo-Sakamoto Y, Akazawa H, Ito K, Takano J, Yano M, Yabumoto C, Naito AT, Oka T, Lee JK, Sakata Y, Suzuki J, Saido TC, Komuro I. Calpain-dependent cleavage of N-cadherin is involved in the progression of post-myocardial infarction remodeling. J Biol Chem. 2014;289(28):19408-19.
- Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114(3):565-71.
- Miwa K, Lee J, Takagishi Y, Opthof T, Fu X, Hirabayashi M, Watabe K, Jimbo J, Kodama I, Komuro I. Axon guidance of sympathetic neurons to cardiomyocytes by glial cell line-derived neurotrophic factor (GDNF). PLoS ONE. 2013:8(7):e65202
- Naito AT, Sumida T, Nomura S, Liu ML, Higo T, Nakagawa A, Okada K, Sakai T, Hashimoto A, Hara Y, Shimizu I, Zhu W, Toko H, Katada A, Akazawa H, Oka T, Lee JK, Minamino T, Nagai T, Walsh K, Kikuchi A, Matsumoto M, Botto M, Shiojima I, Komuro I. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012 149(6):1298-313.

