Multicenter Clinical Study Group
Outline of research and our purpose
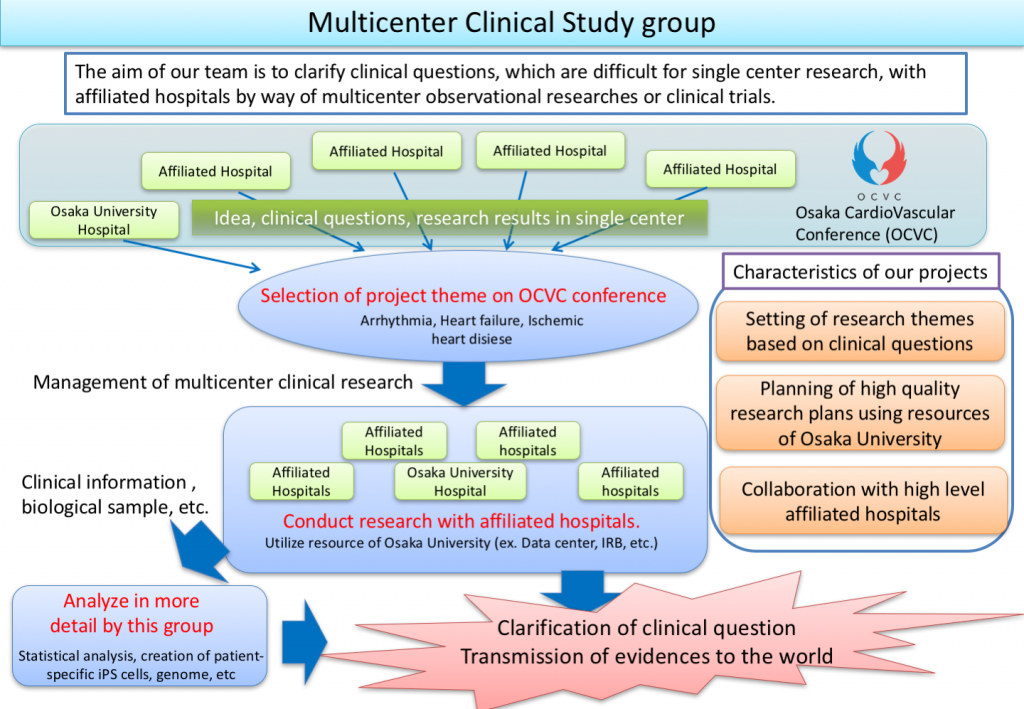
We, Multicenter Clinical Information Analysis Study group, plan clinical researches based on various clinical questions of daily practice and conduct the studies with more than 30 collaborating institutions to offer novel, high-quality information to the world. In the near future, we are going to collect biological samples like blood or somatic cells to find new biomarkers or genes related to pathogenesis or prognosis.
The main works of this group include the following:
- Operational management of multicenter clinical studies,
- Planning of new clinical studies, and
- Analysis of data from clinical studies.
In addition, we are involved in many other research by using biological samples.
Ongoing study
- Registry study of Atrial Fibrillation Patients with Silent Left Atrial Thrombi Detected by Transesophageal Echocardiography
- Multicenter randomized trial regarding catheter ablation for chronic AF (EARNEST-PVI trial : Effect of extensive Ablation on Recurrence in patieNts with pErSistent aTrial fibrillation treated with Pulmonary Vein Isolation)
- Registry study of HFpEF patients (PURSUIT-HFpEF registry: Prospective, MUlticenteR, Observational StUdy of the PatIenTs with Heart Failure with Preserved Ejection Fraction)
- Registry study of AMI patients (OACIS study: Osaka Acute Coronary Insufficiency Study)
For doctoral students,
we can provide you with the chance to learn how to design, manage and analysis clinical study, we welcome those who is interested in clinical study.