心血管再生グループ
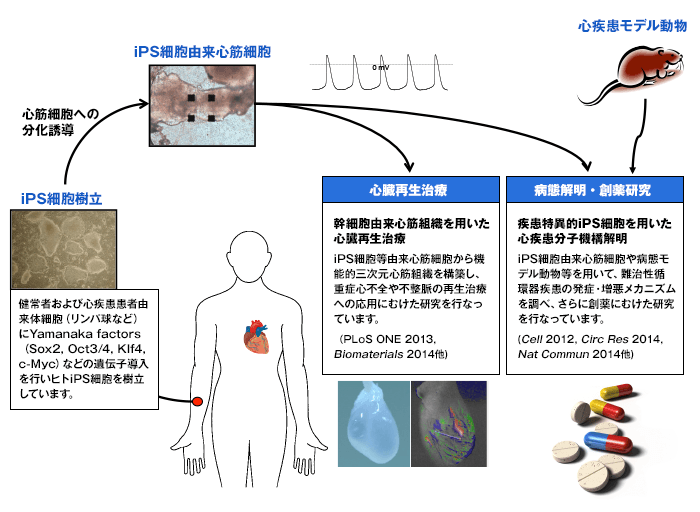
グループ概要
『心臓』を培養皿上で再現することは可能か?
当グループでは、上記問いに答えるために、iPS細胞などの細胞ソースから、組織工学・発生工学的手法を駆使して生体心筋に匹敵する心筋組織の再生を行い、下記研究を行っています:
- ① 心臓再生治療を目指した基盤技術の開発
- ② 疾患特異的iPS細胞を用いた心筋症などの病態解明・創薬
- ③ 疾患モデル動物を用いた病態解明と新規治療法開発に向けた研究
研究目的および内容
研究目的
再生医学的手法を用いて、病態心を培養皿上で再現し、難治性心疾患の病態解明・革新的治療法創出をめざした基盤技術の開発を行うこと。
研究内容
- 心臓再生治療に向けた研究(”Cardiac regeneration therapy”):
iPS細胞/ES細胞から得られた心筋細胞をベースに、組織工学的手法により自律拍動する3次元心臓の構築を行い、生体心に匹敵する再生心筋組織の構築を目指しています。(「3次元バイオ人工心臓・バイオペースメーカの構築」)
さらに、完全房室ブロックモデル動物に、再生心筋組織のin vivo 移植を行い、徐脈性不整脈に対する革新的治療法開発を目指した研究を行っています。
(参考文献: Yoshida et al. Europace 2018 :日本不整脈心電学会で学術奨励賞最優秀賞受賞; Yasui et al. 2014 他)
(https://www.sciencedirect.com/science/article/pii/S0142961214006371?via%3Dihub) - In vitro 病態モデル構築と創薬に向けた研究 (“In vitro disease modeling and Drug discovery”):
健常・疾患特異的iPS細胞を用い、健常心・病態心をin vitroで再現し、疾患メカニズムの解明を行うとともに、創薬に向けたハイスループット評価系や精密アッセイ系を用いて構築しています。(「心疾患の病態を培養皿上で再現する」(“Heart-on-a-dish”))
特に、交感神経細胞や内皮細胞等の非心筋細胞とのクロストークやHippo経路などのシグナル伝達系に着目して研究をおこなっています。(安武秀記:2019日本心筋症研究会YIA優秀賞受賞)
(参考文献:Nakanishi et al. Front Physiol 2019; Kuramoto et al. J Mol Cell Cardiol 2018; Sakai et al. Int Heart J 2018; Hashimoto et al. Int Heart J 2016,) - In vivo 病態モデルの作成とその新規評価法の開発 (“In vivo disease modeling” and “3D-imaging technology”):
心筋梗塞、タコツボ心筋症、完全房室ブロックなどの心疾患モデル動物を用い、特に交感神経リモデリング、線維化、老化、代謝制御等に関わる組織や分子群の3D可視化を可能とするイメージング技術開発を行い、従来の手法では困難であった病態解明に迫る。
(組織透明化技術に基づく3次元イメージング法)
(参考文献: Yokoyama et al. PLoS ONE 2017:ISHR 2016でYIA受賞 他)
(https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0182072)
メンバー紹介
- 【特任教授(常勤)】
- 李 鍾國
- 【招へい教授】
- 日高京子(北九州市立大学 基盤教育センター教授)
- 【特任助教】
- 李 俊
- 【特任研究員】
- 藏本勇希
- 安武秀記
- 【大学院生】
- 増山 潔
- 網屋亮平
- 呂 潔珃
- 【技術補佐員】
- 松島麻悠子
- 小湊智子
- 【連携教員】
- 横山光樹(大阪大学 スポーツ医学講座 助教)
主要論文
- Yasutake H, Lee JK, Hashimoto A, Masuyama K, Li J, Kuramoto Y, Higo S, Hikoso S, Hidaka K, Naito AT, Miyagawa S, Sawa Y, Komuro I, Sakata Y. Decreased YAP activity reduces proliferative ability in human induced pluripotent stem cell of duchenne muscular dystrophy derived cardiomyocytes. Sci. Rep. 2021 May 14; 11(1):10351. doi: 10.1038/s41598-021-89603-8.
- Li J, Lee JK, Miwa K, Kuramoto Y, Masuyama K, Yasutake H, Tomoyama S, Nakanishi H, Sakata Y. Scaffold-Mediated Developmental Effects on Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Are Preserved After External Support Removal. Front. Cell Dev. Biol. 2021. doi: 10.3389/fcell.2021.591754
- Nakanishi H, Lee JK, Miwa K, Masuyama K, Yasutake H, Li J, Tomoyama S, Honda Y, Deguchi J, Tsujimoto S, Hidaka K, Miyagawa S, Sawa Y, Komuro I, Sakata Y. Geometrical Patterning and Constituent Cell Heterogeneity Facilitate Electrical Conduction Disturbances in a Human Induced Pluripotent Stem Cell-Based Platform: An In vitro Disease Model of Atrial Arrhythmias. Front Physiol. 2019
- Kuramoto Y, Naito AT, Tojo H, Sakai T, Ito M, Shibamoto M, Nakagawa A, Higo T, Okada K, Yamaguchi T, Lee JK, Miyagawa S, Sawa Y, Sakata Y, Komuro I. Generation of Fabry Cardiomyopathy Model for Drug Screening Using Induced Pluripotent Stem Cell-Derived Cardiomyocytes from Female Fabry Patient. J Mol Cell Cardiol 2018 Aug;121:256-265.
- Yoshida A, Lee JK, Tomoyama S, Miwa K, Shirakawa K, Hamanaka S, Yamaguchi T, Nakauchi H, Miyagawa S, Sawa Y, Komuro I, Sakata Y. In vitro platform of allogeneic stem cell-derived cardiomyocyte transplantation for cardiac conduction defects. Europace 2018 Sep 1;20(9):1553-1560.
(https://www.ncbi.nlm.nih.gov/pubmed/29554331) - Sakai T, Naito AT, Kuramoto Y, Ito M, Okada K, Higo T, Nakagawa A, Shibamoto M, Yamaguchi T, Sumida T, Nomura S, Umezawa A, Miyagawa S, Sawa Y, Morita H, Lee JK, Shiojima I, Sakata Y, Komuro I. Phenotypic Screening Using Patient-derived Induced Pluripotent Stem Cells Identified Pyr3 as a Candidate Compound for the Treatment of Infantile Hypertrophic Cardiomyopathy. Int Heart J. 2018 59(5):1096-1105.
- Yokoyama T, Lee JK, Miwa K, Opthof T, Tomoyama S, Nakanishi H, Yoshida A, Yasui H, Iida T, Miyagawa S, Okabe S, Sawa Y, Sakata Y, Komuro I. Quantification of sympathetic hyperinnervation and denervation after myocardial infarction by three-dimensional assessment of the cardiac sympathetic network in cleared transparent murine hearts. PLoS One. 12(7):e0182072. 2017
- Iseoka H, Miyagawa S, Fukushima S, Saito A, Masuda S, Yajima S, Ito E, Sougawa N, Takeda M, Harada A, Lee JK, Sawa Y. Pivotal role of non-cardiomyocytes in electromechanical and therapeutic potential of induced pluripotent stem cell-derived engineered cardiac tissue. Tissue Eng Part A. 2017
- Higo T, Naito AT, Sumida T, Shibamoto M, Okada K, Nomura S, Nakagawa S, Yamaguchi T, Sakai T, Hashimoto A, Kuramoto Y, Ito M, Hikoso S, Akazawa H, Lee JK, Shiojima I, McKinnon PJ, Sakata Y, Komuro I. DNA single-strand break-induced DNA damage response causes heart failure. Nat Commun. 8:15104. 2017
- Wang Q, Oka T, Yamagami K, Lee JK, Akazawa H, Naito AT, Yasui T, Ishizu T, Nakaoka Y, Sakata Y, Komuro I. An EP4 receptor agonist inhibits cardiac fibrosis through activation of PKA signaling in hypertrophied heart. Int Heart J. 58(1):107-114. 2017
- Nakagawa A, Naito AT, Sumida T, Nomura S, Shibamoto M, Higo T, Okada K, Sakai T, Hashimoto A, Kuramoto Y, Oka T, Lee JK, Harada M, Ueda K, Shiojima I, Limbourg FP, Adams RH, Noda T, Sakata Y, Akazawa H, Komuro I. Activation of endothelial β-catenin signaling induces heart failure. Sci Rep. 6:25009. 2016
- Takanari H, Miwa K, Fu X, Nakai J, Ito A, Ino K, Honda H, Tonomura W, Konishi S, Opthof T, van der Heyden MA, Kodama I, Lee JK. A new in vitro co-culture model using magnetic force-based nanotechnology. J Cell Physiol. 231(10):2249-56. 2016
- Takanari H, Miwa K, Fu X, Nakai J, Ito A, Ino K, Honda H, Tonomura W, Konishi S, Opthof T, van der Heyden MA, Kodama I, Lee JK. A new in vitro co-culture model using magnetic force-based nanotechnology. J Cell Physiol. 231(10):2249-56. 2016
- Yabumoto C, Akazawa H, Yamamoto R, Yano M, Kudo-Sakamoto Y, Sumida T, Kamo T, Yagi H, Shimizu Y, Saga-Kamo A, Naito AT, Oka T, Lee JK, Suzuki J, Sakata Y, Uejima E, Komuro I. Angiotensin II receptor blockade promotes repair of skeletal muscle through down-regulation of aging-promoting C1q expression. Sci Rep. 25(5)14453. 2015
- Espulgar W, Aoki W, Ikeuchi T, Mita D, Saito M, Lee JK, Tamiya E. Centrifugal microfluidic platform for single-cell level cardiomyocyte-based drug profiling and screening. Lab Chip. 15(17)3572-80. 2015
- Ikeuchi T, Espulgar W, Shimizu E, Saito M, Lee JK, Dou X, Yamaguchi Y, Tamiya E. Optical microscopy imaging for the diagnosis of the pharmacological reaction of mouse embryonic stem cell-derived cardiomyocytes(mESC-CMs). Analyst 140(19)6500-7. 2015
- Yano M, Akazawa H, Oka T, Yabumoto C, Kudo-Sakamoto Y, Kamo T, Shimizu Y, Yagi H, Naito AT, Lee JK, Suzuki J, Sakata Y, Komuro I. Monocyte-derived extracellular Nampt-dependent biosynthesis of NAD(+) protects the heart against pressure overload. Sci Rep. 2015 Nov 2;5:15857.
- Hashimoto A, Naito AT, Lee JK, Kitazume-Taneike R, Ito M, Yamaguchi T, Nakata R, Sumida T, Okada K, Nakagawa A, Higo T, Kuramoto Y, Sakai T, Tominaga K, Okinaga T, Kogaki S, Ozono K, Miyagawa S, Sawa Y, Sakata Y, Morita H, Umezawa A, Komuro I. Generation of Induced Pluripotent Stem Cells From Patients With Duchenne Muscular Dystrophy and Their Induction to Cardiomyocytes. Int Heart J. 2016 Jan 19;57(1):112-7.
- Yamagami K, Oka T, Wang Q, Ishizu T, Lee JK, Miwa K, Akazawa H, Naito AT, Sakata Y, Komuro I. Pirfenidone Exhibits Cardioprotective Effects by Regulating Myocardial Fibrosis and Vascular Permeability in Pressure Overloaded Hearts. Am J Physiol Heart Circ Physiol. 2015 Jun 8: ajpheart.00137.2015.
- Okada K, Naito A.T., Higo T, Nakagawa A, Shibamoto M, Sakai T, Hashimoto A, Kuramoto Y, Sumida T, Nomura S, Ito M, Yamaguchi T, Oka T, Akazawa H, Lee JK, Morimoto S, Sakata Y, Shiojima I, Komuro I. Wnt/b-catenin signaling contributes to skeletal myopathy in heart failure via direct interaction with forkhead box o. Circ Heart Fail. 2015;8:799-808.
- Sumida T, Naito A.T., Nomura S, Nakagawa A, Higo T, Hashimoto A, Okada K, Sakai T, Ito M, Yamaguchi T, Oka T, Akazawa H, Lee JK, Minamino T, Offermanns S, Noda T, Botto M, Kobayashi Y, Morita H, Manabe I, Nagai T, Shiojima I, Komuro I. Complement C1q-induced activation of b-catenin signalling causes hypertensive arterial remodeling. Nature Communications. 2015 (DOI: 10.1038/ncomms7241)
- Kawamura T, Miyagawa S, Fukushima S, Yoshida A, Kashiyama N, Kawamura A, Ito E, Saito A, Maeda A, Eguchi H, Toda K, Lee JK, Miyagawa S, Sawa Y. N-glycans: phenotypic homology and structural differences between myocardial cells and induced pluripotent stem cell-derived cardiomyocytes. PLoS One. 2014 Oct 30;9(10):e111064.
- Yasui H, Lee JK, Yoshida A, Yokoyama T, Nakanishi H, Miwa K, Naito AT, Oka T, Akazawa H, Nakai J, Miyagawa S, Sawa Y, Sakata Y, Komuro I. Excitation propagation in three-dimensional engineered hearts using decellularized extracellular matrix. Biomaterials. 2014;35(27):7839-50.
- Miwa K, Lee J, Takagishi Y, Opthof T, Fu X, Hirabayashi M, Watabe K, Jimbo J, Kodama I, Komuro I. Axon guidance of sympathetic neurons to cardiomyocytes by glial cell line-derived neurotrophic factor (GDNF). PLoS ONE. 2013:8(7):e65202
- Naito AT, Sumida T, Nomura S, Liu ML, Higo T, Nakagawa A, Okada K, Sakai T, Hashimoto A, Hara Y, Shimizu I, Zhu W, Toko H, Katada A, Akazawa H, Oka T, Lee JK, Minamino T, Nagai T, Walsh K, Kikuchi A, Matsumoto M, Botto M, Shiojima I, Komuro I. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012 149(6):1298-313.






